Lithium-Ion Battery Pack
Lithium-ion battery packs are found in a variety of personal electronics, including cell phones, digital cameras and laptops. They also are used in renewable energy systems to store solar and wind power for use when new energy creation isn’t possible.
These batteries have excellent energy density and charge quickly, but can burst into flames if overheated. Learn more about these batteries and how to safely use them.
Benefits
Lithium ion battery technology is rapidly gaining use across a vast range of devices. It has a better energy density than older technologies like nickel metal hydride (NiMH), and it is also much lighter and offers a faster charging rate. It can also withstand hundreds of charge/discharge cycles, Li-ion battery pack and it does not suffer from the memory effect that affects other battery chemistries such as nickel-cadmium.
Using lithium batteries for UPS systems is becoming very popular. They can weigh 40% to 60% less than their VRLA counterparts and have a smaller footprint as well, which allows for a more compact design. They are also able to deliver the same amount of power. This makes them ideal for laptops, smartphones, and other mobile devices.
While there are many benefits to using lithium batteries, it is important to understand the disadvantages as well. They are sensitive to high temperatures and can degrade very quickly if not properly cared for. They are also quite expensive compared to other battery technologies. There is also a small risk that they can burst into flames in some cases.
In order to avoid this, the batteries are designed with a pressure-sensitive vent hole and an overheat protection circuit. They are typically packaged in a metal case and filled with an organic solvent such as ether to exclude moisture. They must also be stored at room temperature because they are not stable in cold conditions.
A lithium battery pack can be damaged by overcharging or discharging, and it must be kept away from direct sunlight as well. It can also be harmed by exposure to excessively high temperatures, which can cause the electrolyte to evaporate and lose its ability to hold a charge. In addition, it can be harmed by shorting or short circuits.
These batteries can be very hazardous if they are exposed to water because it can form lithium hydroxide and hydrogen gas, which are both combustible. They are also not safe to transport by air. Lithium batteries should only be transported in sealed cases and in carry-on luggage, and they must be accompanied by an appropriate emergency plan.
A lithium battery pack must be protected from overcharging and overdischarging by a battery management system (BMS). The BMS is a small computer that monitors the battery’s internal state of charge and prevents it from charging beyond its capacity. It is also a good idea to only charge the battery Li-ion battery pack when it is at or close to full capacity. This will help to extend its lifespan and ensure optimal performance. Top-up charging is also acceptable, but it should not be repeated frequently. It is recommended to charge a lithium battery only about once every two or three months.
Advantages
Lithium-ion battery technology has many advantages over other rechargeable batteries. The most important is that it has much higher energy density, meaning more power in a smaller package. It also allows for faster charging and longer runtimes, and has a better performance in both low- and high-current applications. It can be found in everything from smartphones and laptops to electric vehicles and large-scale energy storage systems.
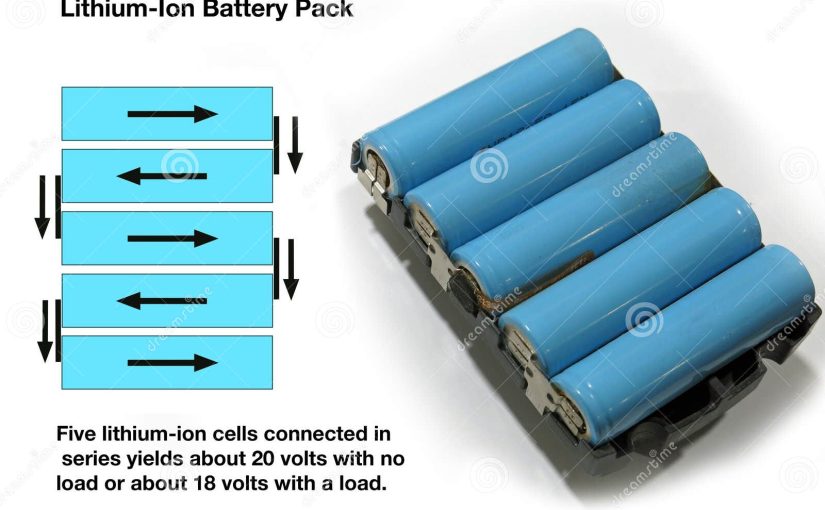
A lithium-ion battery pack typically uses multiple cells, wired together to provide more current than a single cell can handle on its own. The individual cells can have different voltages, but are usually matched to one another to ensure that the total battery voltage stays within safe limits. A protection circuit is also added to help prevent overcharging and under-discharge.
These battery packs can be very small, cylindrical (without terminals) like those used in some e-bike batteries or larger laptop battery cells, or they can be larger and squarer for more high-power applications. They can even be used in a stack to create a custom configuration to fit the design of an application.
There are many different chemistries for lithium-ion batteries, such as lithium cobalt oxide (LCO), lithium manganese nickel cobalt oxide (NMC), or lithium iron phosphate (LFP). NMC and LFP batteries are popular in consumer electronics because of their good cycle life and low cost, while LCO batteries have the highest energy density.
Another advantage of lithium-ion batteries is that they require less maintenance than other chemistries. They do not need prolonged priming when new and only need to be charged a few times to reach their full capacity. They also have very little self-discharge, with a rate less than half that of nickel-based batteries. They do not suffer from memory effect, and they do not need to be completely discharged before recharging.
These batteries are also very safe, with a lower fire risk than most other battery technologies and no hazardous waste issues when disposed of. However, they must be kept out of extreme heat to avoid degrading quickly. They are subject to aging, even if they are never used, so it is important that the manufacturing date and an accurate storage temperature be considered when purchasing them.
It is also possible for lithium-ion batteries to explode, though this is very rare and only occurs in two or three battery packs per million. This is because they are so sensitive to temperature and can be triggered by a variety of conditions. To reduce this risk, it is recommended that the batteries are stored in a cool place and always at least 40% charged. This will help slow the aging process.