Lead Acid Battery Service

If you’re wondering about lead acid batteries and how to care for them, you’ve come to the right place. Learn about their construction and maintenance, as well as their life expectancy. To get started, follow these tips: Make sure the battery is charged and that the electrolyte level is at the top of the plates.
About lead acid batteries
Many people are unsure about the proper lead acid battery service. This is a complex and often mysterious process. There are numerous factors that can shorten the lifespan of this type of battery. Regardless of the cause, the best way to extend its life is to properly charge and discharge the battery. The following are some tips to help you get the most life out of your battery.
First, charge your lead acid battery to full capacity as soon as possible. If your battery is not fully charged, you will permanently reduce its capacity. This is because lead acid batteries contain large crystals of lead sulfate, which are unlike the porous lead electrode. This makes it harder to break down the crystals and convert them back to lead.
Another way to extend the life of your battery is to check the fluid levels periodically. These fluid levels should not be too low or too high. This will help prevent your battery from developing problems and premature failure. It’s best to use distilled water to fill your lead acid battery. Also, remember to not store batteries in direct sunlight.
A lead acid battery goes through three distinct phases. The first is the formatting phase, where the plates are surrounded by liquid electrolyte. When you exercise the plates, the electrolyte is absorbed by the electrodes and increases capacity. A deep cycle battery can deliver between 100 and 200 cycles before it begins to decline. However, it is important to replace it before the capacity drops below 70 percent.
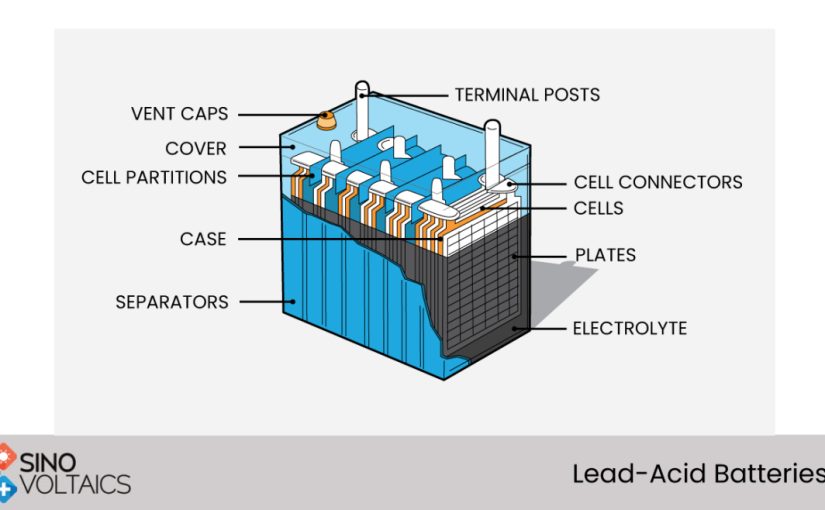
Construction
Lead acid batteries come in a variety of designs. These batteries are categorized according to their construction, and are typically made up of two main components: a grid and lead. The grid plays a critical role in the lead-acid battery’s conductivity, as it ensures that currents are distributed evenly across all the active components. If currents are not spread evenly, the battery can lose its ability to function properly.
Lead acid batteries come in flat, cylindrical, or tubular plates. These are mass-produced by either casting or wrought techniques. The active material is then applied to grids, which are then dried. The larger, industrial lead-acid batteries usually use tubular plates, which have longer cycle lives. They are also more protective of the spines that carry the current.
The lead-acid battery is often the most popular storage battery type, and is widely used for a variety of applications. It is made up of cells made of lead, with each cell composed of plates immersed in an electrolyte. This electrolyte is dilute sulfuric acid. The electrolyte helps the battery store energy, and it is also the main component of a lead-acid battery.
The lead-acid battery has been used for decades. It is strong and reliable, and has a very long life span compared to other battery types. Understanding how it works lead acid battery service is essential for maximizing its performance and extending its life. Once you know the basics, you can properly maintain a lead-acid battery.
Maintenance
Proper maintenance of lead acid batteries is essential to their performance and lifespan. Even the most basic maintenance can make a big difference in the performance and efficiency of your batteries. If you want to extend the life and reduce your operating costs, consider switching to lithium-ion batteries. There are six essential maintenance tasks that you should do regularly to maintain your lead-acid batteries.
First, check the terminal connectors of the battery. They should be tight and free from corrosion. If there is any corrosion, cover them with petroleum jelly. Also, check for warpage, which is a sign of overcharging or overheating. Also, do not overfill the battery as this could result in an overflow of electrolyte. If you use water to top up your battery, be sure to use distilled or demineralised water. Never use sulphuric acid to refill your battery.
Another important maintenance tip for lead acid batteries is to make sure the fluid level is right. If it is too high or too low, your battery will not be able to function properly. Battery manufacturers recommend that you check the fluid level regularly to avoid premature failure or damage. Also, it is important to use distilled water when you top off your lead acid battery. By following these steps, you can extend the life and capacity of your batteries.
Another crucial step is to make sure the battery room is properly ventilated. The water in lead acid batteries will break down into hydrogen and oxygen gas, which are both flammable. For this reason, it is important to make sure the battery room is well-ventilated and that people are using protective clothing.
Life expectancy
The lifespan of lead acid batteries depends on how often they are charged and discharged. If you don’t discharge them by more than 5 percent, they can last for about 25 years. However, if you do discharge them too much, they won’t last as long as they could have. This is why it’s important to keep a record of your discharge and recharge cycles.
To maximize the life of your lead acid battery, charge it before and during storage. Always keep the charging voltage below the gassing voltage. This will avoid lead acid battery service damaging the battery. Once you charge a lead acid battery properly, it will return to a nominal state over time. However, if you regularly discharge it beyond its capacity, it could become permanently damaged.
As the charge and discharge rates increase, the capacity of lead acid batteries decreases. This phenomenon is called Peukert’s law. This means that the higher the load, the faster the battery will discharge, and the lower its capacity. When a lead acid battery has a high current load, its capacity can fall as low as 60%.
Another factor that affects the life expectancy of lead acid batteries is temperature. A battery is liable to lose capacity if the temperature is too cold or too hot. In addition, high temperatures accelerate the aging process of the battery.
Common failures
A battery’s performance degrades over time due to the electrochemical reaction it undergoes. This degradation is not always apparent. There are several reasons for a battery to fail, and none of them is immediate. In some cases, batteries may not even show signs of failure. One exception to this is thermal runaway.
One of the main reasons for a battery to fail is due to a short circuit. These short circuits may occur for a number of reasons, including a damaged separator or a bent plate. The damaged separator will cause the negative and positive plates to become connected. This will increase the internal impedance, reducing the capacity of the battery. Impedance testing can indicate if a battery is experiencing a short circuit.
Another reason for a battery to fail is a lack of water. As water in the battery vaporizes, it loses capacity. This process is also called vulcanization. In order to prevent this from happening, you must add pure water and bring the battery back to its normal capacity. After doing this, you must seal the battery slot with adhesive. This will reduce the chances of vulcanization. Corrosion of the positive grid is also another reason for a battery to fail. The anodic oxidation reaction damages the electrical contact between the active material and grid.
In addition to excessive cycling, the improper connections between the batteries can result in battery failure. The wrong connections can also cause poor battery performance and charging inefficiency. These are some of the reasons why lead acid batteries are susceptible to premature failure.
Remedies
Remediations for lead acid battery service often involve adding certain chemicals to the electrolyte. These chemicals dissolve the lead sulfate that has been deposited on the plate and help improve the battery’s overall performance. This practice has been around since the 1950s and is a useful stopgap measure for aging batteries. However, these additives do not solve the underlying problem of corroded plates or damaged separators.
The traditional explanation of sulfation is that the battery plates become covered in a layer of lead sulfate, which prevents the battery from delivering power. When this layer is removed, the battery will be able to restore its original capacity and power. But the traditionalists have overlooked an important flaw in their theory.
Whenever a lead acid battery is discharged, it releases electrons. The electrolyte contains a large concentration of lead sulfate, which reacts with the lead to produce a sulfate. As the acid level decreases, the lead sulfate begins to fill the battery plates. This reduces the flow of electrolyte and lowers the battery’s voltage. Eventually, the battery’s electrolyte content will fall to levels that are too low to store any energy.
A flooded lead acid battery requires special maintenance, including regular checking of the electrolyte levels and the specific gravity. When used in a vehicle, a battery should be checked every 15 minutes after it has been boosted or equalized. Aside from regular maintenance, flooded batteries should also undergo “boost charging” if possible.