A Review of Lithium Iron Phosphate Batteries

LFP batteries are a type of battery that uses lithium iron phosphate as the cathode material. These are very similar to lithium ion batteries, but they offer several advantages. They offer better energy density, longer lifespan, and less chances for chemicals to leak into the environment.
Lithium-iron-phosphate (LiFePO4)
If you are looking for a more efficient and eco-friendly power source for your home, then you may want to consider lithium iron phosphate batteries. These types of batteries are safer and stronger than other lithium batteries. This makes them ideal for a wide variety of applications.
Lithium iron phosphate is a new type of battery that was first developed in the early 2000s. It is a better alternative to other lithium chemistries because it has a lower cost and can be manufactured with less energy.
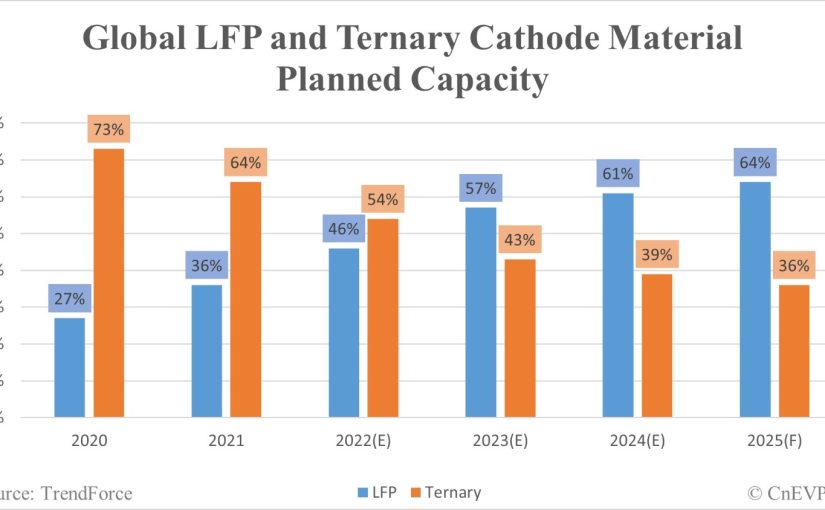
Although these batteries aren’t yet in widespread use, they are gaining popularity in the manufacturing industry. They can be used in a variety of products, from electric vehicles to radio equipment and emergency lighting.
The advantages of this new technology include high capacity and low self-discharge. Also, it is able to handle a higher charging current. In addition, it produces little heat during its lifetime.
Since it is non-combustible, it is safer than other lithium batteries. The lithium-ion batteries can catch fire in hazardous situations, but the lithium iron phosphate is non-toxic and won’t explode. Compared to lead acid batteries, it is lighter and has a longer runtime.
Depending on the temperature, the lithium iron phosphate discharges at a rate of 1-25C. At higher temperatures, the cells can be discharged to a depth of up to 80%. With a life of up to 5,000 charge/discharge cycles, they are rated to last about 10 years.
Another advantage of this technology is that it can be made from recycled materials. This helps to reduce waste. Moreover, lithium iron phosphate is recyclable and can be recovered from batteries. Because it isn’t toxic, it is also environmentally friendly.
While lithium iron phosphate is still considered a new battery, it is an excellent choice for consumers and manufacturers. It is more environmentally safe and less expensive than lead acid batteries. And, because of its lower weight, it can also be used in a wide variety of applications. Whether you are an electrician or a homeowner, you can benefit from these powerful and affordable batteries.
Higher energy density than lithium-ion
While lithium-ion batteries have dominated the high-energy battery market for decades, several systems have emerged with lower energy densities. These alternatives, while lfp batteries often less expensive, are generally poor cyclability and face numerous challenges. This review discusses the advantages and disadvantages of each alternative and examines their suitability for certain applications.
The first step in the design of a high-energy battery is to select an electrode material. Materials available for use include insertion electrodes, conversion electrodes, and metallic anodes. Using a periodic table, one can assess the suitability of each material for a given application.
In addition to ion charge capacity, the energy density of an electrode is directly related to its redox potential. In general, higher atomic masses of transition metal elements result in lower theoretical energy densities. However, this trend is not as significant in anodes as in cathodes.
Sodium is a relatively cheap, alkali metal like lithium. Like lithium, it is abundant in the earth’s crust. It has a similar chemical structure to lithium, and has been investigated for use as a insertion anode. Although its ionic radius is larger, the increase in charge storage capacity is not as large.
Aluminium is also a promising candidate as an insertion anode. A trivalent aluminium ion has a low standard reduction potential, but a high surface charge density. As a solid element, it is lower in solubility than lithium. It has been investigated as a replacement for lithium, but has not yet been used commercially.
Other materials, such as zinc and magnesium, have much lower energy densities. Zinc is a good choice for aqueous electrolytes, but it has a significantly lower charge capacity than lithium. Magnesium, on the other hand, is cheaper than lithium, but has a slightly lower charge capacity.
Another option for an insertion anode is phosphorus. Phosphorus is a low-cost ion, but is generally unsuitable for conversion reactions with metallic cations at room temperature.
Silicon is another candidate for an insertion anode. Silicon has a redox potential of 0.4 V against metallic lithium. If it were to be used as an insertion anode, it would require significant structural changes.
Longer lifespan than traditional li-ion
One of the benefits of lithium-ion batteries is their ability to last for a long time. They have a much higher energy density than any other type of battery technology, and they also require a lot less maintenance. The key is to care for your battery properly. It can be a huge investment for your business, so it’s important to make sure it lives as long as possible.
Fortunately, researchers have found a way to make high-voltage lithium-ion batteries last lfp batteries for a much longer period. In fact, they could extend their lifespan three times! This is a major advancement that could be immediately applied to the electric vehicle industry in China.
Specifically, this new technology replaces a chemical solution with a gel-like substance. In addition, the process is scalable. These types of batteries can be recharged in as little as two minutes, and can last up to 70 percent longer than traditional batteries.
Researchers are also working to develop new, more efficient lithium-ion batteries. They’ve also found ways to improve safety and reduce the risk of a fire.
An international team of scientists has just published a technique that could greatly increase the lifespan of lithium-ion batteries. They discovered that certain materials can prevent the cathode from dissolving. Moreover, the specific material is relatively cheap.
The study was conducted by a group of Chinese and American researchers, led by Associate Professor Li Nianwu at Nanyang Technology University. This work was also published in the peer-reviewed journal Advanced Functional Materials last week.
While the technique is still experimental, the results are promising. A lithium-ion battery with a titanium anode would be able to last 20 years or more, while a graphite anode would only last five.
However, the research still needs further development. For example, scientists are looking at how different materials can change the shape of the battery. Since the anode and cathode are made from different materials, the difference in the material can have a big impact on the life of the battery.
In addition, researchers are working to improve the cost and flexibility of lithium-ion batteries. As the demand for electric vehicles increases, the need for more battery power will only grow.
Less likely to leach chemicals into the environment
When batteries are disposed of improperly, they can cause pollution and damage to the environment. Depending on the type of battery, they can release toxic gases, hazardous chemicals, or vapours. However, when LFP (lithium-ion phosphate) batteries are discarded, they are less likely to release chemicals into the environment. This is a result of the fact that LFP cells contain phosphate salts that are less soluble than metal oxides in other lithium batteries.
In addition to the pollutants released by these spent batteries, they can be hazardous to human health and wildlife. The study identifies the potential hazards of these batteries and their disposal. It discusses how they may be contaminated and how they can be prevented from polluting the air, water, and soil.
The main sources of waste from LIBs include recycling and landfilling. Recycling is the process of taking the battery apart and using it again, usually for another application. Lithium batteries are a good source of energy, but they can also pose a threat to human and animal life.
Improper disposal of batteries can lead to contamination of the environment and fire risk. They also release hazardous pollutants and VOCs. Despite the potential dangers, there are a number of current practices used to dispose of spent LIBs. These practices include collection, landfilling, recycling, and illegal disposal.
There is a need to improve battery management systems to ensure safe and environmentally friendly disposal. But before that can be accomplished, there are some knowledge gaps to address. One such gap is the understanding of the processes and pathways involved in the environmental impacts of spent batteries.
Several studies have been conducted to assess the environmental effects of various LIB chemistries. Some of these studies have been funded by the UK’s Engineering and Physical Sciences Research Council.
These studies highlight the importance of proper storage and recycling. However, they are not sufficient to fully examine the impact of EoL LIBs. As a result, the current review aims to identify the most likely risks and suggest actions to mitigate them.
For instance, in the UK, large LIBs are treated as hazardous materials. They can be thrown in a landfill or sent to mainland Europe for recycling. Nevertheless, storing and disposing of old batteries is a complicated process that can be costly and inefficient.